Quality Control at VetBac: How We Validate Every Batch
Quality Control at VetBac: How We Validate Every Batch
When a new batch of ready-to-use agar plates is delivered and placed into storage, we randomly select several plates from that batch for QC testing. These plates are then tested using standard disc diffusion methodology, in accordance with EUCAST quality control principles.
Depending on the scope of the QC run, we may perform:
- Short QC checks (e.g., one bacterial species with a basic antibiotic panel), or
- Extended QC experiments involving multiple species and antibiotics
This flexibility reflects real diagnostic use while still maintaining control over analytical performance.
Which bacteria do we use — and why?
Our routine QC panel includes four bacterial species (Figure 1):
- Escherichia coli – representing Enterobacterales and the most common cause of urinary tract infections in dogs and cats
- Pseudomonas aeruginosa – a non-fermenting Gram-negative rod with distinct growth and resistance characteristics
- Staphylococcus aureus – representing staphylococci, the most common pathogens in skin and ear infections
- Enterococcus faecalis – often present as secondary pathogens, but clinically relevant due to intrinsic and acquired resistance
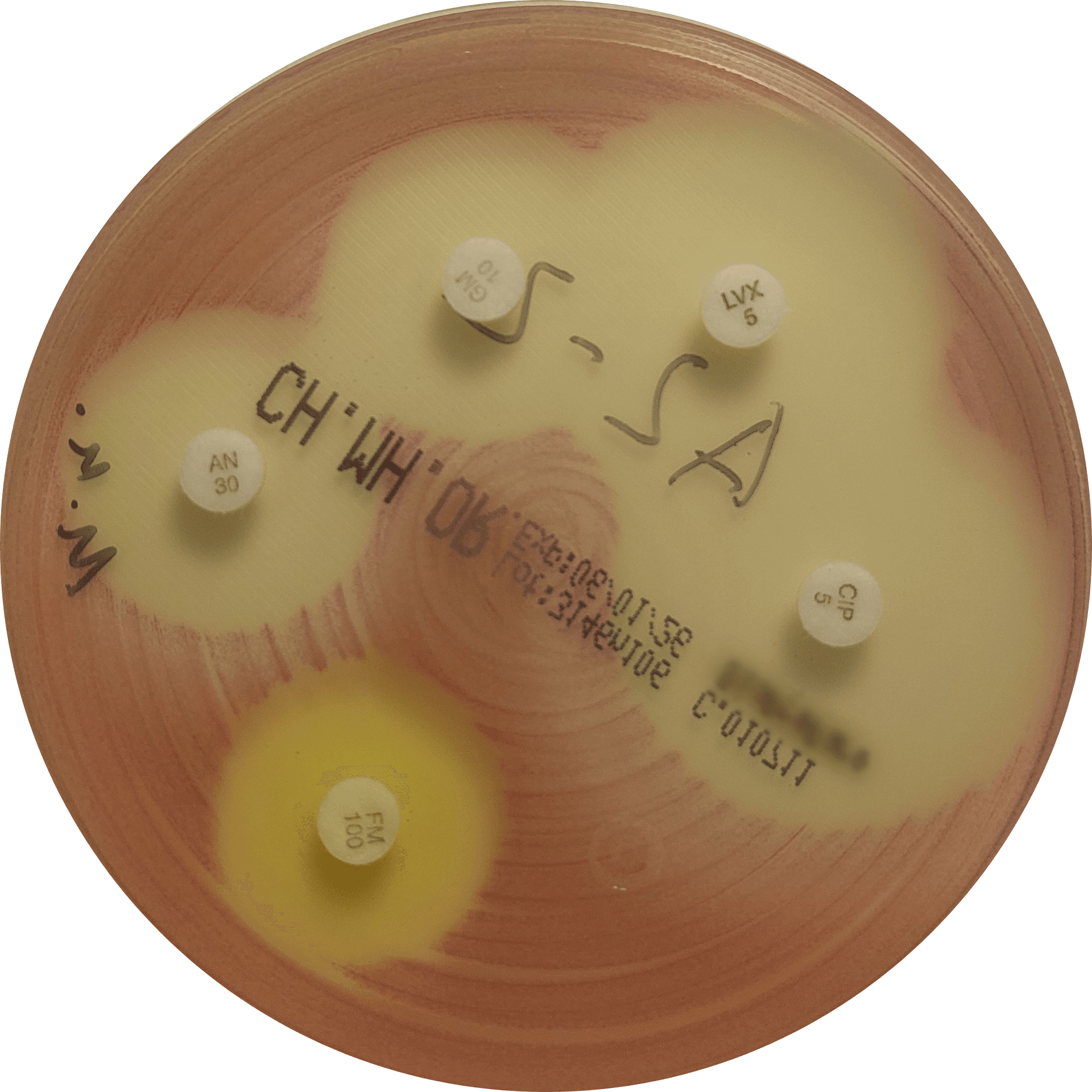
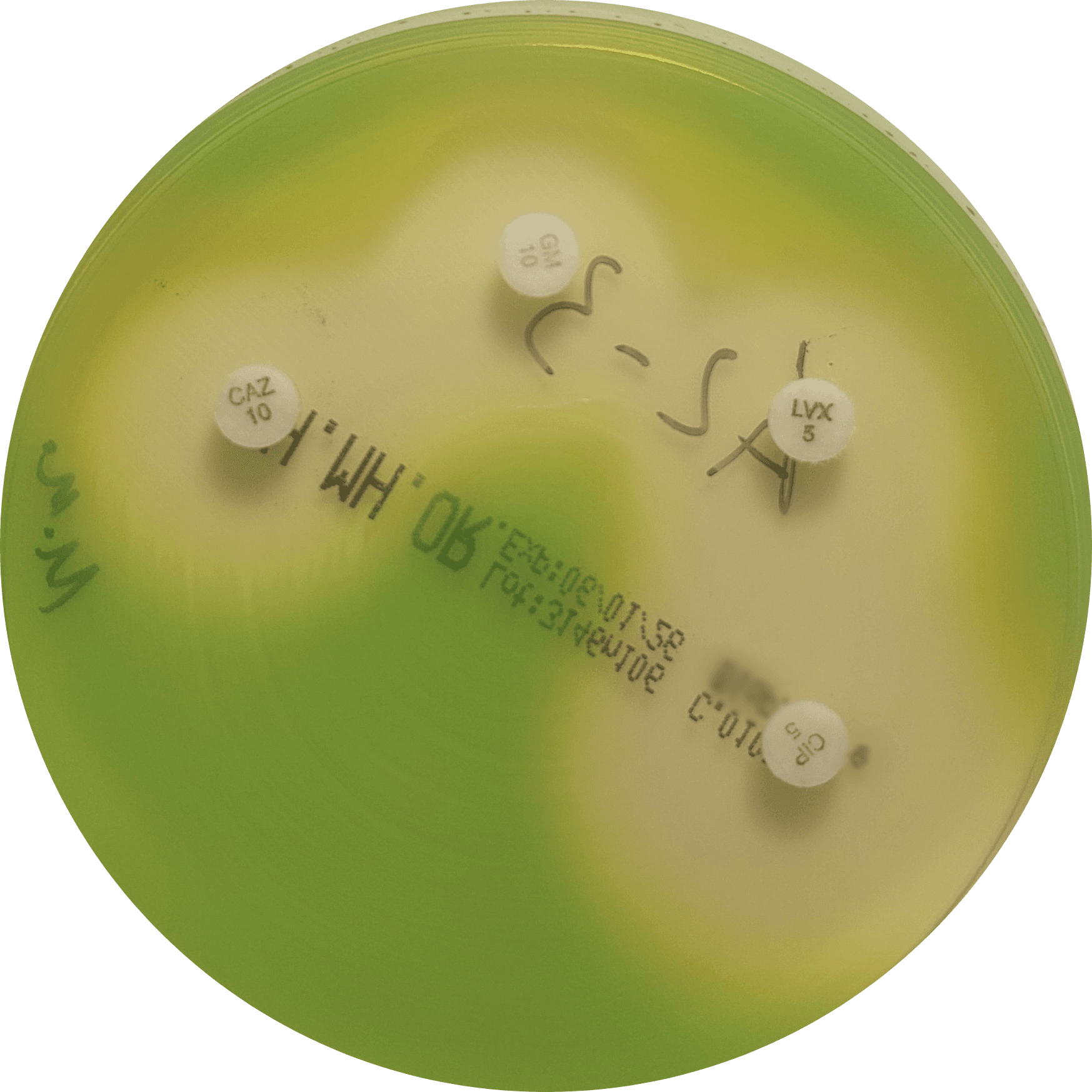
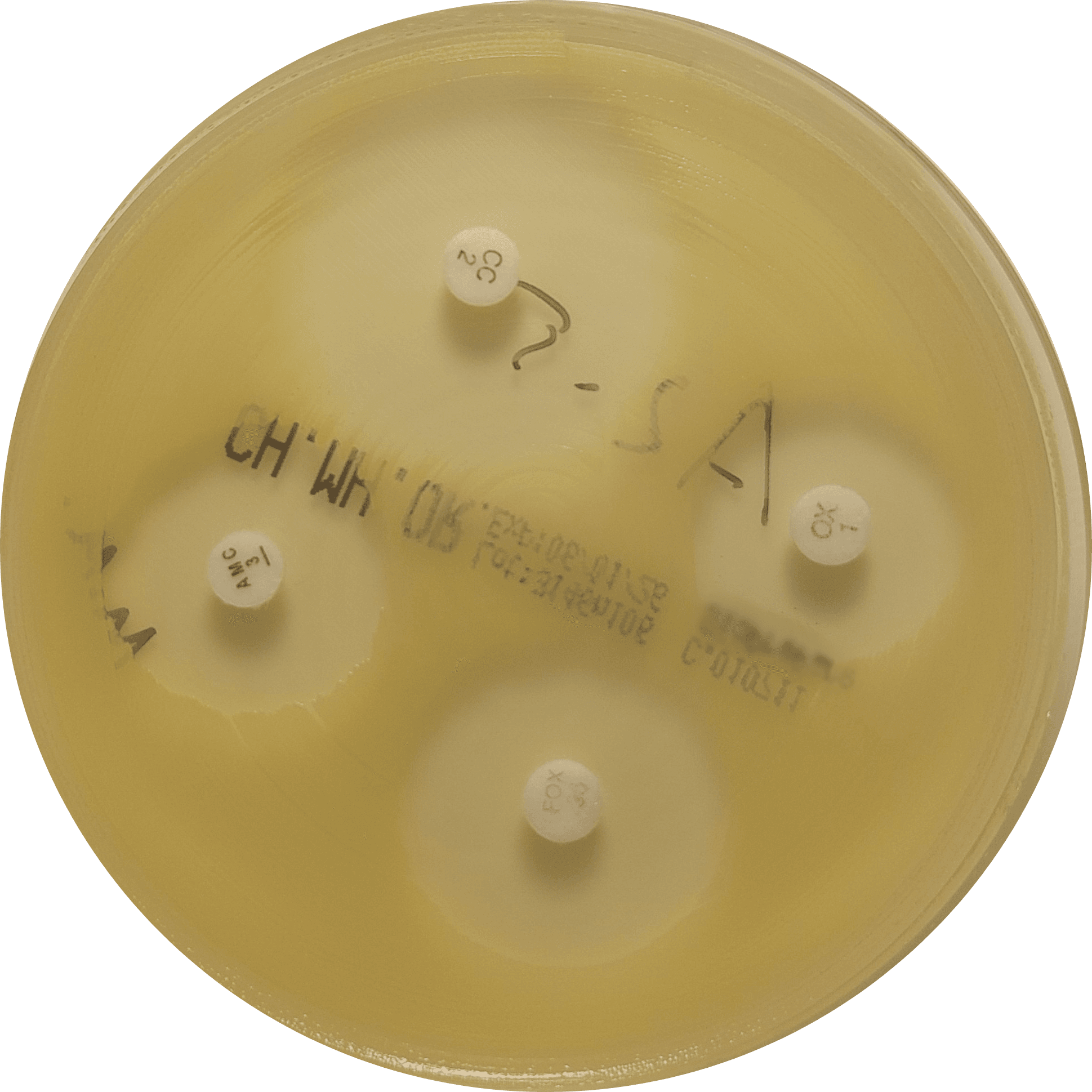
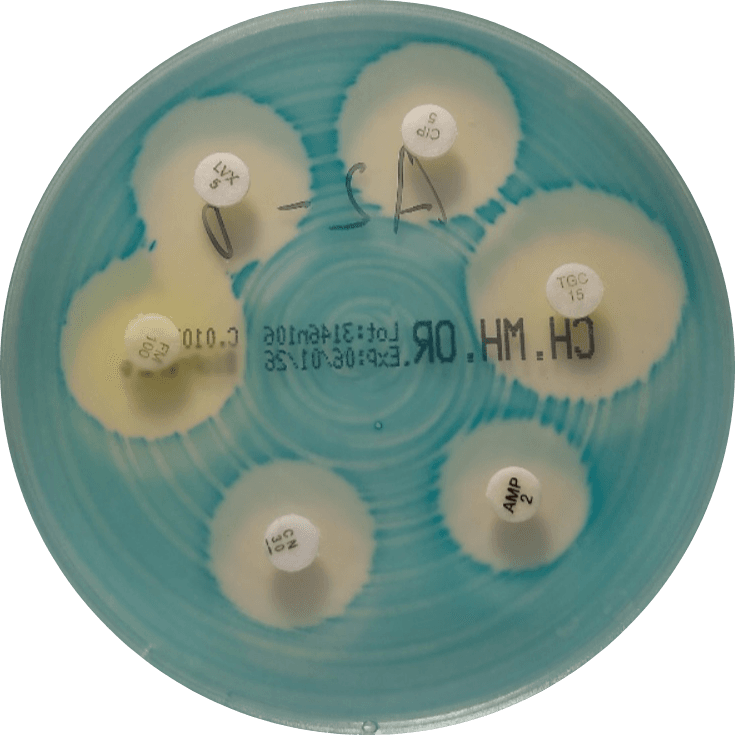
Figure 1. Representative growth of quality control organisms on VetBac agar plates: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis.
Together, these organisms represent the major bacterial groups encountered in routine small-animal practice, including Gram-negative rods and Gram-positive cocci, fast- and slow-growing bacteria, and bacteria with different antimicrobial susceptibility profiles.
How is performance evaluated?
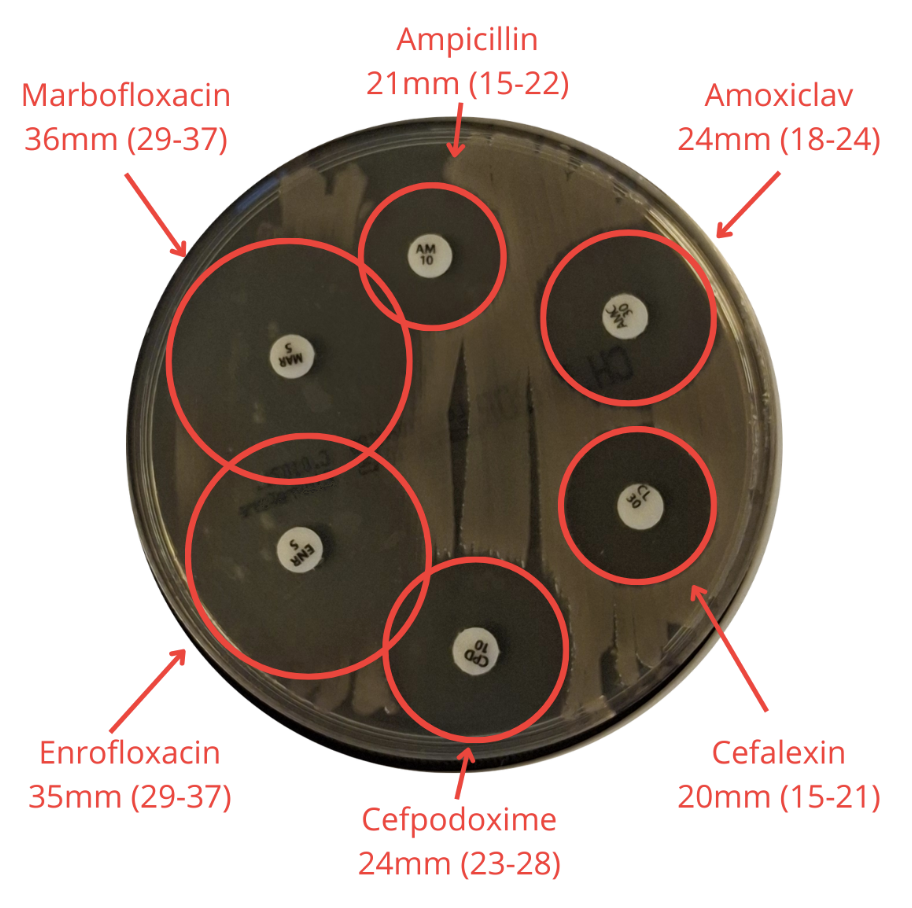
For each QC run, inhibition zone diameters are measured (Figure 2) and compared against EUCAST QC ranges and target values. This allows us to verify that:
- The agar supports adequate bacterial growth
- Antibiotic diffusion behaves as expected
- Measured zones fall within accepted EUCAST limits
- Any deviation triggers further investigation before plates are used clinically
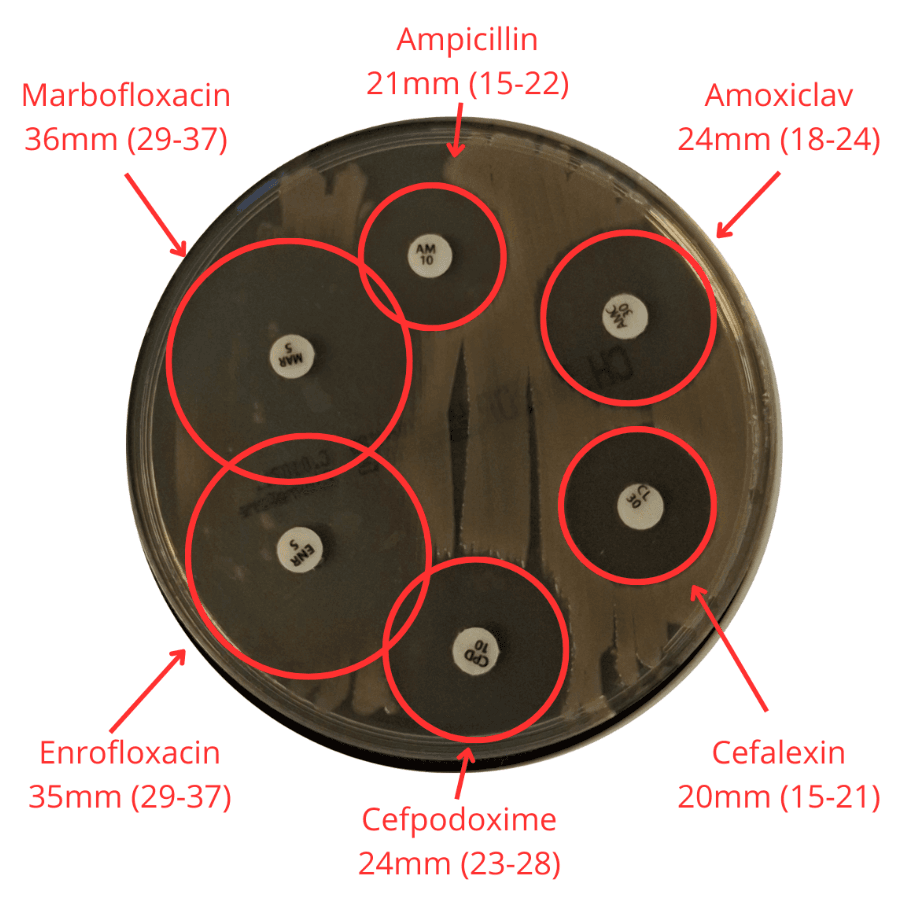 Figure 2. Measurement of inhibition zone diameters during quality control testing.
Figure 2. Measurement of inhibition zone diameters during quality control testing.
Linking QC to real-world veterinary diagnostics
While EUCAST QC guidelines are defined under standardized laboratory conditions, VetBac is designed for use in veterinary clinics rather than reference laboratories. Our QC strategy, therefore, focuses on confirming robust performance under conditions that realistically reflect in-clinic testing, while remaining anchored to EUCAST methodology.
This approach ensures that clinicians can trust the results generated overnight in their own practice, while still knowing that the underlying system is continuously monitored and validated.
Evidence and transparency
Our quality control work is ongoing. During 2026, we plan to publish several QC-related studies evaluating the performance of our media under EUCAST-aligned conditions.
To support transparency and informed clinical use, these studies will be shared on this page as soon as they become publicly available. We believe that openly communicating QC data is an essential part of responsible antimicrobial susceptibility testing and antimicrobial stewardship in veterinary medicine.
Explore More Veterinary Insights
Ready to improve your antibiogram testing?
Get overnight results with clear, actionable reports.
Book a demo